서브비쥬얼
Acanthopanax senticosus Acanthopanax Senticosus
서브 컨텐츠
Acanthopanax senticosus
Hambakjae Bio Farm use superior natural materials to develop innovative products to provide health solutions
R&D
R&D
In the last 15 years, we studied 15 different kinds of medicinal plants and one of them that we mainly focused on research was Acanthopanax.
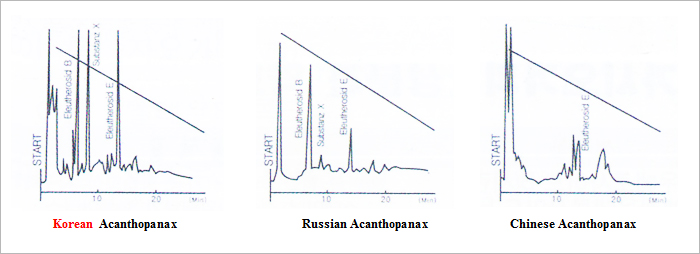
Wagner (Dr. H. Wagner) had studied more than 20 different Korean medicinal plants
(including Acanthopanax) for more than one decade and since 1982 he started to
publish study results about Acanthopanax. Prof. Wagner compared the differences of
Acanthopanax from South Korea, China, and Russia and the different content of
Eleutheroside E and B in each country’s Acanthopanax.
Research result showed that content of Eleutheroside E and B in Korea’s Acanthopanax is
respectively four times and six times compare to China’s and Russia’s. It proved the superior
effects of Korean Acanthopanax as a medicinal plant.
- Experimental Result Figures
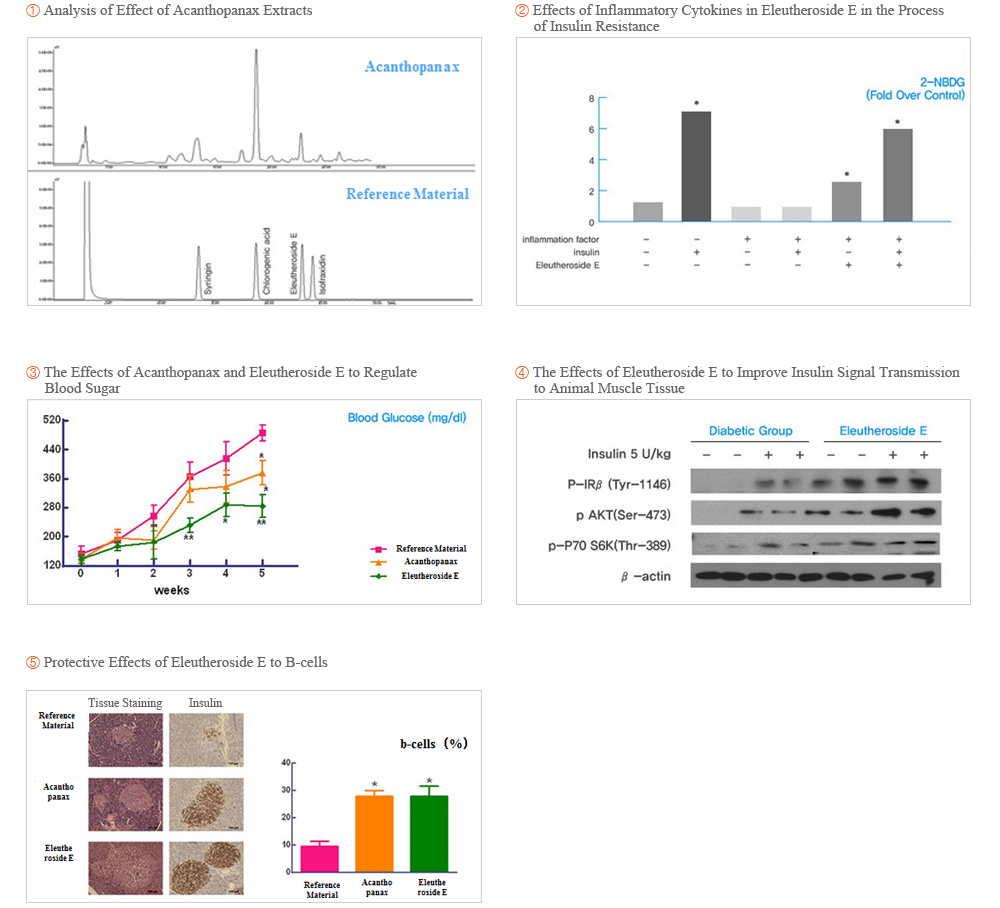
Korea Food Research Institute Korea Food Research Institute’s study results showed that the Korea Acanthopanax Eleutheroside E improves insulin resistance and has diabetes treatment effects
- ① Korea Food Research Institute’s study indicated that the main component of Acanthopanax Eleutheroside E is effective to improve insulin resistance. Insulin resistance means that the body's insulin-mediated glucose uptake and metabolism reduction, including insulin sensitivity and reactivity decreased, it is also a feature of obesity. Korea Food Research Institute found that Eleutheroside E can improve Insulin-induced glucose uptake in muscle cells, improve insulin resistance caused by the tumor necrosis factor -α (TNF-alpha). In the stimulation of insulin signal to promote glucose into muscle tissue.
- ② Registered patent of component that contains Eleutheroside E to prevent or treat insulin resistance (Patent No.: 1015362310000,Registered Date: 7th July 2015 ).
- ③ Hambakjae have been selected as an ‘2013 Human applicable clinical trials supporting enterprise for functional food evaluation’ by Ministry of Agriculture, Food, and Rural Affair and Korea Food Research Institute. With financial support of 200 million Korean won from the government, clinical trials to test the effect of Acanthopanax in control blood sugar is being carried out. With the result of trials, we plan to apply for individual certification of individual type of health material from Ministry of Food and Drug Safety.